Chemistry of Paints
Chemistry of Paints
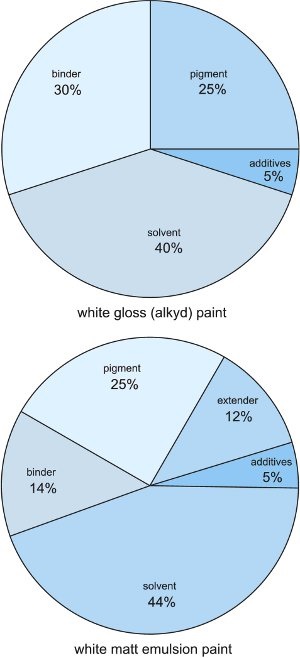
This unit discusses the most commonly used binders followed by the pigments.
Important Binders (Resins) in Modern Paints?
The three most important binders (resins) used in modern paints are:
- acrylic polymers (resins)
- alkyd polymers (resins)
- epoxy polymers (resins)
Acrylic polymers (resins)
The binder in many emulsion paints is based on homopolymers or co-polymers of ethenyl ethanoate (vinyl acetate) and a propenoate (acrylic) ester.
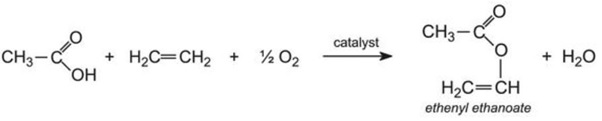
Ethenyl ethanoate is manufactured by passing a mixture of ethanoic acid vapour, ethene and oxygen over heated palladium(ll) and copper(ll) chlorides:
Ethenyl ethanoate and an acrylic ester (for example, methyl 2-methylpropenoate) are then co-polymerized to form a random array, in which these groups link into a linear chain:
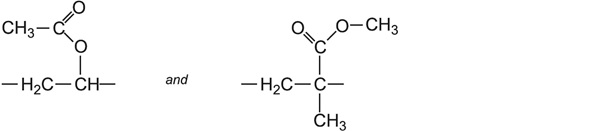
Other acrylic esters used as co-monomers with ethenyl ethanoate are ethyl propenoate, butyl propenoates, or a co-polymer of butyl propenoate and methyl 2-methylpropenoate.
The polymers used in these paints are carried in water (water-borne emulsion paints) which as described above are much better for the environment than paints in which the binders are in organic solvents.
Emulsion paints are so-called as they are made by a process known as emulsion polymerization, in which the liquid monomers to be polymerized are first dispersed in water, as an emulsion. The polymers produced by this process typically have relative molecular masses of 500 000 – 1 000 000. As such they are useful only as dispersions since they would be extremely viscous if they were carried in solution and this would make them unusable.
Acrylic resins may also be used in industrial paints, either as water-borne emulsion paints or as solvent-borne paints. Solvent-borne industrial paints can have a tough protective finish and are widely used in industry as topcoats, for example on car bodies. The paint frequently comes as two components which are mixed together just before use: the main paint portion typically consists of an acrylic resin produced by the polymerization of a propenoate ester formed from a polyhydric alcohol (diols and triols). The resulting polyester has numerous hydroxyl groups (-OH) pendant from the polymer backbone. The hydroxyl groups react with the other compound often consisting of a polymeric isocyanate such as a trimer of 1,6-diisocyanatohexane (hexamethylene diisocyanate):
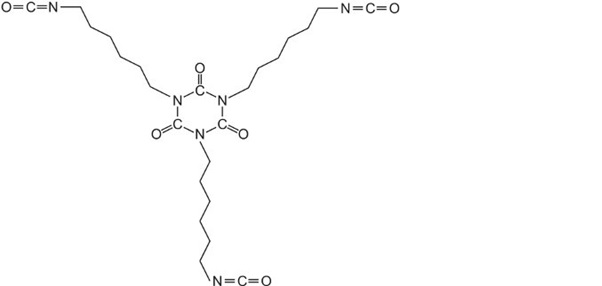
Such a compound is known as a crosslinker for it produces, on reaction with the resin, a three-dimensional structure similar to the polyurethane formed from a polyol and an isocyanate.
When these two components are mixed together, a chemical reaction takes place between the hydroxyl groups on the polymer (acrylic resin) and the isocyanate groups on the cross linker:
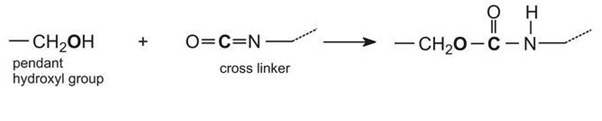
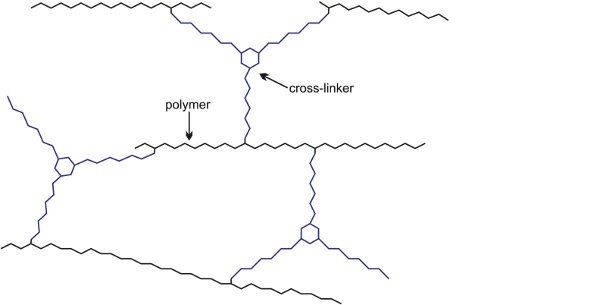
This reaction proceeds relatively slowly at room temperature, allowing enough time for the paint to be applied, after which the solvent thinner evaporates and the painted item is placed in an oven to accelerate the chemical reaction. This greatly increases the molecular mass of the polymer causing it to become a three-dimensional molecule and form a hard film, resistant to chemicals
Alkyd polymers (resins)
Decorative gloss paints typically contain alkyd polymers (resins). A typical resin is that produced from a polyol such as propane-1,2,3-triol (glycerol) with a dibasic acid such as benzene-1,2-dicarboxylic (phthalic) anhydride and a drying oil (linseed or soybean oil). On being heated together, ester linkages are formed, and water is a by-product. The name alkyd is derived from alcohol and anhydride.
The first step in making the alkyd polymer is the reaction between the triol and the drying oil to produce a monoglyceride. For example:
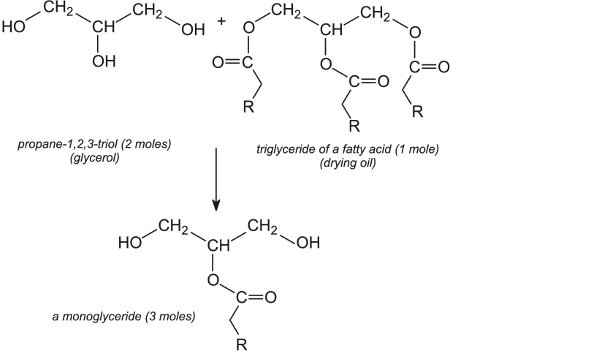
The monoglyceride then reacts with the anhydride to form the alkyd polymer (resin):
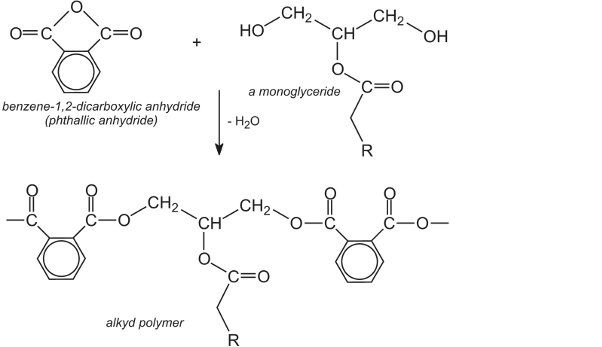
The alkyd resins, which generally have relative molecular masses in the range of 10 000 – 50 000, are usually carried in organic solvents (solvent-borne paints). Turpentine extracted from trees was used in the past as the solvent, but this has been replaced by solvents from petrochemical feedstock, such as `white spirit` which is a mixture of aliphatic and alicyclic hydrocarbons.
Once the alkyd resin is applied, the pendant oil drying groups react with oxygen in the air to form a cross-linked, hard thermoset coating, with a high molecular mass.
Epoxy polymers (resins)
Epoxy resins are often used as the binder in industrial coatings (primers). They give the paint excellent adhesion together with high resistance to chemicals (corrosion), and physical resistance necessary, for example, on ships and chemical storage tanks.
The epoxy resins are made from 1-chloro-2,3-epoxypropane (produced from 3-chloropropene) and substituted phenols, such as bisphenol A:
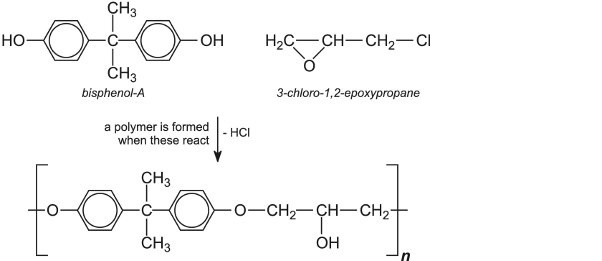
The value of n can be controlled to give a range of resins varying from viscous liquids to solids with high melting points. Epoxy resins can be carried in solvents such as aromatic hydrocarbons, alcohols, ketones and esters (solvent-borne paints) or as dispersions in water (water-borne paints) as true emulsions. They are not normally used in topcoats for outdoors because they are susceptible to UV degradation, but they make excellent interior coatings and exterior primers.
Epoxy resins are also used as adhesives (e.g. Araldite) and electrical insulators.
Pigments used in paints
Paint drying
- Source: http://www.essentialchemicalindustry.org
