Galvanneal
What is Galvanneal?
In manufacturing galvanneal, the main differences from Hot Dipped Galvanized in the process are that a lower level of aluminum (typically 0.11% to 0.14%) is used in the zinc bath, and the moving, zinc-coated strip is reheated immediately after it passes the air-wiping step above the zinc bath. By heating the strip to between 935 – 1050°F [500 – 565°C], and holding for a few seconds, the zinc coating, by way of diffusion, alloys with the iron in the steel. The end result is that the coating is converted into layers of zinc-iron intermetallic compounds with a bulk composition of approximately 90% zinc and 10% iron. These are averages, as the iron percentage varies throughout the coating thickness, from as low as ~6% at the surface, to as high as ~23% at the steel interface. Galvanneal coatings have no free-zinc present and have a low-lustre matte appearance, versus the metallic sheen of galvanized coatings. The final bulk iron concentration depends mostly on the heating cycle, since the rate of diffusion is primarily a function of time and temperature. The zinc and steel chemistries can also affect the alloying behavior, but they are secondary to the heating cycle.
The differences in characteristics and performance between galvanneal coatings versus galvanize coatings are explained below.
Coating Composition
A galvanize coating is essentially pure zinc, with between 0.20 and 0.50% bulk aluminum, although the aluminum is mostly concentrated in the thin inhibition layer next to the steel. The aluminum is present, not to affect the corrosion performance, but to enable good coating adhesion, which is required when the coated sheet is eventually formed.
Galvanneal coatings contain 9-12% bulk iron, along with the above-mentioned aluminum, which becomes more uniformly spread through the coating thickness than in the case of galvanize. The iron in galvanneal coatings is definitely not uniformly distributed, however, as it is combined with zinc in 3 distinct zinc-iron phases. These phases are shown in Table 1, along with their iron and aluminum contents.
| Table 1 Galvanneal Phases and Compositions | ||
| Alloy Layer | % Fe | % AI |
|
Zeta (z) FeZn13 |
5.2 - 6.1 | 0.7 |
| Delta (d) FeZn10 | 7.0 - 11.5 | 3.7 |
| Gamma (G) Fe3Zn10 | 15.8 - 27.7 | 1.4 |
The phases in Table 1 are shown in the order that they occur in the coating, with the high-iron gamma layer next to the steel substrate. It is also important for good appearance and press formability that the top zeta layer contain no less than about 5% iron or there will be a risk of free zinc on the surface. The higher bulk aluminum content through the three alloy layers is a result of its diffusion outward from the inhibition layer next to the steel where it was concentrated prior to the reheating cycle.
Galvanneal coatings are hard and brittle, so as a result of bending and press forming, coating cracks and powdering are always present to some degree. Each coating line must develop practices to produce the optimum coating properties for the particular end use, in order to balance performance in forming versus coating line throughput.
The phases in Table 1 are shown in the order that they occur in the coating, with the high-iron gamma layer next to the steel substrate. It is also important for good appearance and press formability that the top zeta layer contain no less than about 5% iron or there will be a risk of free zinc on the surface. The higher bulk aluminum content through the three alloy layers is a result of its diffusion outward from the inhibition layer next to the steel where it was concentrated prior to the reheating cycle.
Galvanneal coatings are hard and brittle, so as a result of bending and press forming, coating cracks and powdering are always present to some degree. Each coating line must develop practices to produce the optimum coating properties for the particular end use, in order to balance performance in forming versus coating line throughput.
Production of Galvanneal
The moving sheet is immersed in the zinc bath and a thin, aluminum-bearing, inhibition alloy layer forms at the zinc/steel interface. As the strip emerges from the bath it drags excess zinc with it, which the air knives remove to obtain the desired coating weight. The strip, with the still liquid zinc coating, enters the galvanneal furnace about 10-15 feet above the gas wiping knives. Before the zinc can solidify, reheating of the strip begins. As the strip temperature rises, the zinc-iron diffusion reaction restarts and breaks down the aluminum-zinc-iron inhibition layer that formed in the zinc pot at the steel zinc interface. After 57 seconds of heating, and up to about 10 seconds of soaking, enough iron diffuses into the coating to convert it to a dull matte gray appearance. Diffusion in liquid zinc is faster than in solid zinc, alloying the proper alloying reactions to occur during this short time.
Figure below is a schematic representation of the galvannealing process.
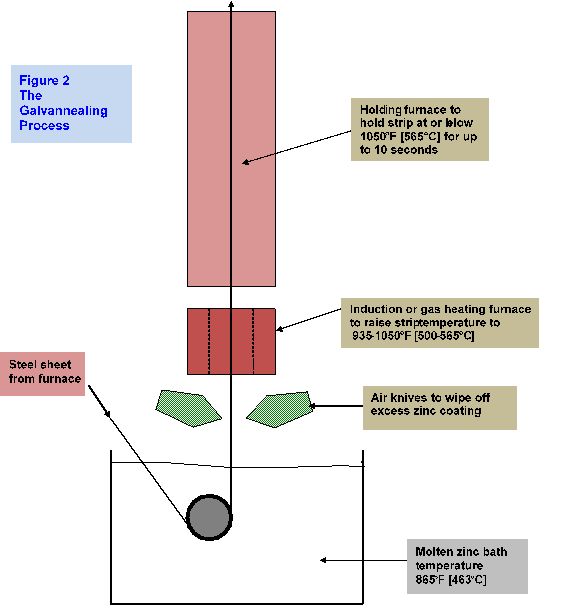
Figure 2 The Stages of Alloying Between Steel Sheet and Molten Zinc Coating to Produce “Galvanneal”
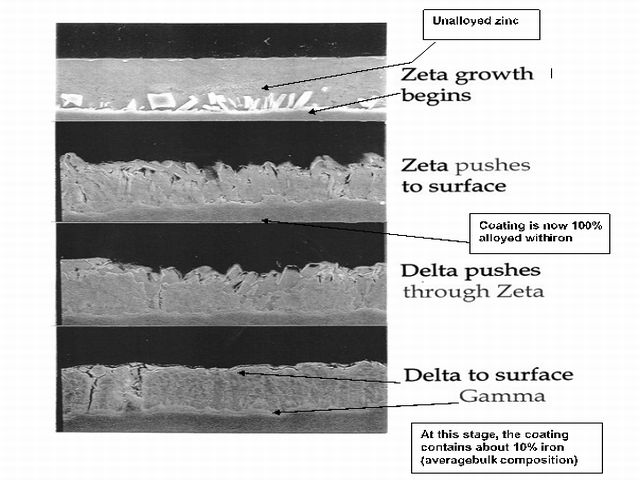
Figure 2 The Stages of Alloying Between Steel Sheet and Molten Zinc Coating to Produce “Galvanneal”
What are the benefits of Galanneal over Galvanize?
The benefits of using galvanneal over galvanize are:
- Improved spot-weldability
- Ease of painting and improved coating adhesion
Weldability – Zinc-iron alloy coatings generally have better spot welding characteristics than pure zinc coatings.1 The coating’s higher electrical resistance, along with its higher hardness and higher melting point, allow good welds to be obtained at lower currents with longer electrode life, to the extent that galvannealed sheet spot-welds very much like cold rolled sheet.
Paintability – Performance of galvanneal under paint is synergistically improved partly because of the excellent bond formed between the paint and the surface of the coating. The reason for the good bond is evident in Figure 4 – the paint can “mechanically lock” with the zinc-iron crystals on the surface, in addition to any chemical bonding that may occur. This is why galvanneal coatings can be painted directly without the need for a primer. Compared to galvanize, galvanneal generally exhibits less undercutting corrosion beneath paint at exposed edges, scratches, or other breeches in the paint. To achieve maximum corrosion protection, galvanneal is usually treated with zinc phosphate before painting (all auto bodies made with galvanneal are zinc phosphate treated prior to painting).
Formability – A galvanize coating is quite soft, and easily scratched. A galvanneal coating is very hard, and thus not as easily scratched when handling. The harder zinc-iron alloy powders on deformation, unlike pure zinc coatings, which can gall and flake.2
The good frictional behaviour and ductility of zinc, combined with the excellent adhesion achieved between the coating and the steel, allows galvanized sheet to be formed into many intricate shapes without any loss in coating adhesion. In fact, because the coating is soft, care needs to be exercised to prevent flaking resulting from galling.
Adherence – Even though the galvanneal alloying reaction results in a hard, relatively brittle coating, it can be bent, stretched and drawn when correct sheet manufacturing and part forming procedures are used. Many parts made from galvannealed sheet require a deep drawing operation. When deep drawn, galvannealed sheet typically exhibits some “powdering” of the coating as a result of high compressive strain that can occur during the forming operation. By proper control of the steel manufacturers’ processing practices, combined with the proper setup of the drawing dies, and the use of appropriate drawing lubricants, the amount of powdering can be minimized and excellent performance can be achieved.
The powdering of a galvannealed coating during forming is a function of many parameters, mostly relating to the steel manufacturing practices covered previously. The most important coating characteristic affecting the powdering tendency is the coating thickness. The amount of powdering rises directly as the thickness increases. For this reason, the maximum coating weight for galvanneal is restricted to A60 (0.60 oz/ft2) [ZF180 (180 g/m2)]. For many applications, an A60 coating weight is too thick to provide acceptable powdering performance, and many users specify A40 (0.40 oz/ft2) [ZF120 (120 g/m2)], or less.
In fact, most automobile applications of galvanneal use the equivalent of about an A30 [ZF90] coating. The tendency for A60 to powder should be considered when selecting a coating weight.
The second largest influence on the adherence of galvanneal coatings is the percent iron in the coating.
The bulk iron level must be within a range of 9-12% to perform satisfactorily in most forming operations. Iron levels of less than 9% result in softer coatings that can contain free zinc at the surface. This will change the coefficient of friction and interfere with drawability and stretchability of the sheet. Iron levels over 12% result in harder coatings that can create excessive powdering, to the point of fouling forming dies. Some users of galvanneal prefer iron levels near either the upper or lower end of the above range due to the nature of their forming processes. Producers of galvanneal must understand how to control coating iron levels on their equipment in order to provide satisfactory product for the range of their customer base.
Generally, there are no significant differences in the properties of the steel substrate, whether it is galvanize or galvanneal. Any differences in forming behaviour (splits, etc.) are usually related to the different nature of the two metallic coatings. For example, the substantial difference in coating hardness can necessitate changes to the stamping parameters, i.e., die type, die clearances, hold-down forces, lubrication type, etc.
What is the Corrosion performance of Galvanneal?
The thickness of a galvanize coating has a direct influence on the corrosion performance and life of the product, i.e., the thicker the coating, the longer its life.
The corrosion performance of a galvannealed coating is more complicated than its galvanized counterpart. Almost all applications of galvannealed sheet involve painting after fabricating. The primary reason is that, when unpainted, the presence of 10% iron in the coating can quickly lead to a “reddishcolored” corrosion product when the surface becomes wet. Research has shown3 that the color is related mainly to corrosion of the iron within the coating and does not necessarily signify that corrosion of the steel substrate is occurring. This discoloration is purely a cosmetic effect, but precludes the use of unpainted galvanneal in severe corrosion environments, as users find it to be unacceptable.
As most applications for galvanneal are painted after fabrication, corrosion studies of galvanneal are in its painted state, meaning that there is no data on the corrosion performance of galvanneal compared to bare (unpainted) galvanize. The importance of the galvanneal coating thickness is often revealed at sheared edges or scratches, etc, i.e., places where the steel and metallic coating are directly exposed to the corroding environment. At such discontinuities, a thicker coating can improve resistance to “undercutting” the paint film, i.e., a thicker galvanneal coating can slow down degradation of the paint, as evidenced by edge “creep back” corrosion and eventual total loss of paint adhesion. To maximize service life, it is therefore advisable to use as thick a galvanneal coating as potential powdering problems will allow.
Considering the relative corrosion rates:
- A pure zinc coating (galvanize) provides a high degree of galvanic protection to exposed steel such as at sheared edges and scratches.
- A galvannealed coating is about 10% less galvanically active in most environments because it contains 10% iron. Its bare corrosion rate may, in fact, be less than pure zinc but is masked by the “reddish-colored” corrosion products that can form on the surface in the presence of water. Keep in mind that galvanneal will maintain its original matte grey appearance if it never gets wet.
- The more galvanically active galvanize coatings could be quickly consumed when acting as a galvanic protector to any exposed steel. The less galvanically active galvanneal coatings do not offer as much galvanic protection, and therefore are not as rapidly consumed during the corrosion process. It is interesting to note that automotive galvanneal coatings equivalent to about A30 have performed just as well as approximate G50 galvanize coatings with respect to the corrosion resistance of painted outer auto body panels. This superior field performance also was found in salt fog testing4 where painted galvanneal was found to be more resistant to underfilm corrosion than painted hot-dipped or electrogalvanized steels.
- When painted galvannealed steel is exposed to an environment with less wetness, the difference in performance between it and painted hot-dipped or electrogalvanized steel is not as significant.5 • The specific needs of the application and the corrosion performance requirements dictate which coating will perform best. Other requirements for the application, such as weldability and the specific capabilities of each product manufacturers’ paint shop need to be considered when deciding which product is best for a given situation.
Where to use Galvanneal?
Galvanneal is widely used for commercial doors and frames. They are easily field painted with no adherence problems. Interior doors and frames can be left unpainted if desired. Galvanneal surfaces exposed to the weather must be painted to avoid corrosion.
When considering whether to use galvanize or galvanneal for a specific application, find the answer to such questions as:
- What are the corrosion demands of the end use and the environment? Coating thickness is the primary consideration.
- Is spot welding involved? Galvanneal will perform better in terms of welding tip life.
- Is the product going to be used unpainted? In most cases, galvanize is preferred, but galvanneal can be used in the unpainted state for interior applications.
- Is deep drawing involved? Before using galvanneal, stamping trials may need to be conducted in order to assure that the degree of powdering is acceptable.
In almost all applications, there is more than one issue involved. The correct product recommendation by the producer requires in-depth consideration of all processing steps included in manufacturing, plus knowledge of end-use requirements.
- Source: www.galvainfo.com
